When developing a new product, we call for collaboration with small/medium size business that understand the market needs well. We can support not only the development of a new product, but also other process of medical device business –from consulting, system build-ups, manufacturing, and to market release throughout—in one place. That’s our strong point, and, we believe, it will eventually result in cost-down.
By providing our “one-stop solution” service, J-sol Medical will strive for maximizing our clients’ satisfaction. We like to support new commers to the medical device industry as well as existing companies that already import medical devices but wish to develop in-house products for lowering risks.
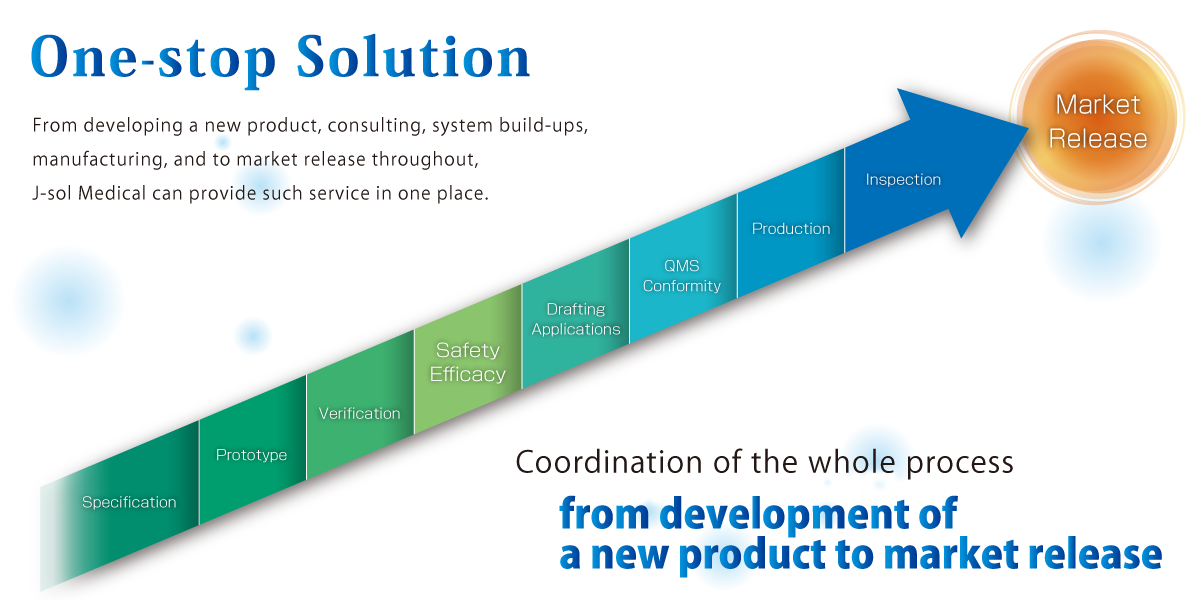
Case Example of “One-Stop Solution”
- Development Phase:Specification
- Clients’ requests hearing to decide specification of a product.
- Development Phase:Prototype
- According to the specification, a prototype can be made at our laboratory. At J-sol medical, it has equipped a 100,000 class (ISO5) cleanroom. Outsourcing of making a prototype with our partner companies is also an alternative.
- Development Phase:Verification
- We conduct efficacy measurement for medical device. When in outsourcing a trial, we can support liaisons with various test institutions.
- Regulatory Assistance Service:Safety/Efficacy
- -support liaisons with test institutions for electrical, biological and other safety testing.
- Regulatory Assistance Service:Drafting Applications
- -draft registration applications and prepare for inquiries from PMDA.
- Regulatory Assistance Service:QMS Conformity
- -review SOP and various record forms to support completion of QMS compliance inspection by PMDA.
- Manufacturing
- -support transferring of manufacturing flow based on QMS Ordinance to a client’s company or other designated manufacturing site.
- Quality Inspection
- -carried out by our staff who receives training regularly. Products which have passed the inspection then will be packed and labelled accordingly with QMS operation procedures.
- Release into Market
- -in order to release medical device domestically, it must obtain permission from a proper marketing authorization holder in Japan.