Regulatory Assistance
Since its establishment in 1997, J-sol medical has provided consulting services related to drafting registration applications for medical device imports , submitted to Ministry of Health, Labour and Welfare.
Our experience can help small/medium size companies tackle various problems they face when entering medical device business. As a license holder for manufacturing and 1st class marketing authorization, we have worked out many cases regarding QMS compliance inspection;we can present an optimal solution for a client who may find it difficult to deal with SOPs, etc.
Providing one-stop solution for medical device, we hope to serve our clients’ needs in every possible way.
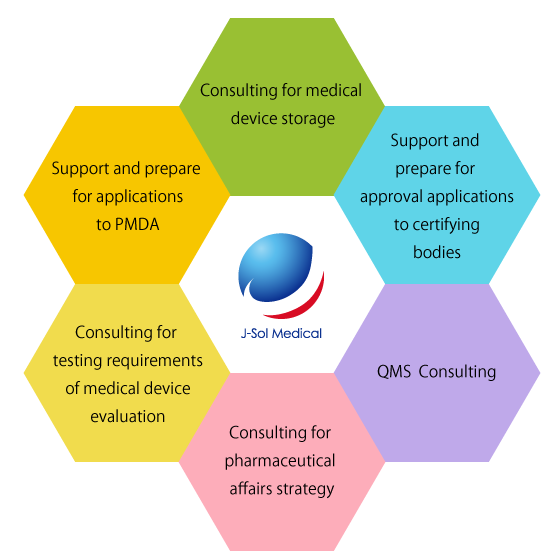
Support and prepare for approval applications to certifying bodiesFrom the preparation of approval application to certifying bodies required for the sales of medical device to acquisition of approval afterwards, we support them all. |
Support and prepare for applications to PMDAWe provide full-range supports for applications to authorities, from preparing for notification to acquisition of approval afterwards, necessary for the sales of medical devices. |
QMS ConsultingFor those clients involvied in design/development, it is obligated to receive QMS audit from either certifying bodies or PMDA. We help prepare for documents needed for the audit. |
Consulting of pharmaceutical affairs strategyAdvise the most efficient way to enter the medical device market. |
Consulting of testing requirements for medical device evaluationHelp you with the testing requirements for the evaluation of efficacy and safety of medical device. We may also recommend some safety testing institutions. |
Consulting of medical device storageOur warehouse is a Registered Manufacturer class required for storing medical devices. |
From registration documentation to post-marketing, we can provide full-range services.
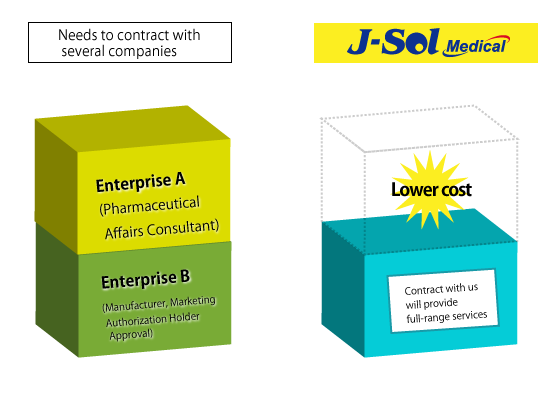 As medical device related businesses often require extensive expertise; that is why medical devices related companies are divided into different domains such as companies for preparing applications/ approvals, companies for storing medical devices, and companies for supporting the QMS audit.
As medical device related businesses often require extensive expertise; that is why medical devices related companies are divided into different domains such as companies for preparing applications/ approvals, companies for storing medical devices, and companies for supporting the QMS audit.
We contribute to our customers by providing a total solution for medical devices so that customers can reduce their cost by combing all the operations through our services. Our staff who are fluent in English will support communications with overseas companies for you.
Accomplishment in regulatory assistance
Entrusted operations for clinical trials
Coronary Stent, Ophthalmological Laser Irradiation Device, Hyperthermia Device for Bladder Cancer, 3T MRI
- Drafting IB, designing protocol, planning CRF
- Monitoring
- Data entry and statistical analysis
- Clinical study report
- Preparing SOP and GCP audit
GMP and QMS audit
- Radiopharmaceuticals and In vitro diagnostic agent
- Marketing authorization for medical device
- Marketing authorization for medical device
Drafting applications for marketing approval of medical device such as:
Types of medical devices
Cardiovascular
- Coronary Stent
- PTCA product
- Artificial lung
- Electrocardiograph
- Implanted cardiac pace maker
- Cardiopulmonary pump
- External Defibrillator
- Cardiac output flowmeter, Esophagus catheter
Orthopedics
- Autologous blood device
- Bone cement
- surgical instrument(KMC)
Neurosurgery
- Surgical navigation system
Ophthalmology
- Laser equipment for photodynamic therapy
Anesthesiology/Intensive Care
- Artificial respirator
Radiology
- 3T MRI
- Medical electron accelerator
Others
- Leucocyte removing filter
Post-marketing Research
Coronary Stent, Laser irradiation apparatus for photodynamic therapy
Mechanical Stress Resistance Test, Planning and Performing Preclinical Safety Test such as accelerated fatigue test
- PTCA catheter, Tensile strength test for coronary arteries, Pressure proof test
- Leakage current test for electrocardiograph, temperature measurement operation, Power input test
Drafting applications for certifying medical devices such as:
- Medical electrical apparatus
- Single-use device
- Anesthesia/Respirator apparatus
- Ophthalmological/Visual apparatus
- Radiation/Diagnostic imaging apparatus
- Hearing aid
- Home electric massager/Electric therapeutic apparatus for home use and its related equipment
- Others
Consulting service related to establishing QMS system, Drafting QMS related documents
- Assist to establish QMS based on MHLW Ministerial Ordinance No.169
- Assist to establish QMS based on ISO13485,








